Ksp - Solubility product constant definition
Solubility product constant is simplified equilibrium constant (Ksp) defined for equilibrium between a solids and its respective ions in a solution. Its value indicates the degree to which a compound dissociates in water. The higher the solubility product constant, the more soluble the compound.
The Ksp expression for a salt is the product of the concentrations of the ions, with each concentration raised to a power equal to the coefficient of that ion in the balanced equation for the solubility equilibrium.
Ok - so what does that mean?
Solubility product constants are used to describe saturated solutions of ionic compounds of relatively low solubility. A saturated solution is in a state of dynamic equilibrium between the dissolved, dissociated, ionic compound and the undissolved solid. As for everu solution, at equilibrium in given conditions we can write an expression like the one below for Silver chloride:
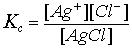
Where [Ag+] and [Cl-] represent concentrations of ions of Ag+ and Cl- and [AgCl] is an value representing an amount of moles in a litre of solid AgCl. [AgCl] is a constant, therefore, writing a following equation:
Kc x [AgCl] = [Ag+][Cl-]
we can notice that product of equilibrium concentration of Ag+ and Cl- is equal to a constant. This constant is called solubility product constant or Ksp
Ksp = [Ag+][Cl-]